Naegleria fowleri
This article is missing information about genome assemblies (drafts and one unpublished but finished genome on NCBI). (January 2021) |
| Naegleria fowleri | |
|---|---|

| |
| Diagram depicting the stages of Naegleria fowleri's life-cycle and environment at that stage | |
| |
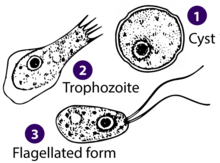
| Drawings of the three stages Naegleria fowleri's life-cycle | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Percolozoa |
| Class: | Heterolobosea |
| Order: | Schizopyrenida |
| Family: | Vahlkampfiidae |
| Genus: | Naegleria |
| Species: | N. fowleri
|
| Binomial name | |
| Naegleria fowleri R.F.Carter, 1970
| |
Naegleria fowleri, also known as the brain-eating amoeba, is a species of the genus Naegleria. It belongs to the phylum Percolozoa and is classified as an amoeboflagellate excavate,[1] an organism capable of behaving as both an amoeba and a flagellate. This free-living microorganism primarily feeds on bacteria but can become pathogenic in humans, causing an extremely rare, sudden, severe, and almost always fatal brain infection known as naegleriasis or primary amoebic meningoencephalitis (PAM).[2]
It is typically found in warm freshwater bodies such as lakes,[3] rivers, hot springs,[4] warm water discharge from industrial or power plants,[5] geothermal well water,[6] and poorly maintained or minimally chlorinated swimming pools with residual chlorine levels under 0.5 g/m3,[7][8][9] water heaters,[10] soil, and pipes connected to tap water.[11] It can exist in either an amoeboid or temporary flagellate stage.[12]
Etymology
[edit]The organism was named after Malcolm Fowler, an Australian pathologist at Adelaide Children's Hospital, who was the first author of the original series of case reports (British Medical Journal, starting 1965) of PAM.[13][14]
Life cycle
[edit]
Naegleria fowleri, a thermophilic and free-living amoeba, is primarily found in warm and hot freshwater environments such as ponds, lakes, rivers, hot springs, and poorly maintained swimming pools.[15] As temperatures rise, its population tends to increase. Although the amoeba was initially identified in Australia in the 1960s, it is believed to have evolved in the United States.[16] N. fowleri exists in three forms: cyst, trophozoite (ameboid), and biflagellate. While it does not form cysts in solid human tissue, where only the amoeboid trophozoite stage is present, the flagellate form has been discovered in cerebrospinal fluid.
Cyst stage
[edit]To endure harsh environmental conditions, trophozoites transform into microbial cysts,[17] spherical, single-layered structures about 7–15 μm in diameter, enclosing a single cell nucleus.[18] Acting as a resilient capsule, the cyst enables the amoeba to withstand adverse circumstances. Factors triggering cyst formation include food scarcity, overcrowding, desiccation, waste accumulation, and cold temperatures. When conditions improve, the amoeba can emerge through the pore or ostiole at the center of the cyst. N. fowleri has been observed to encyst at temperatures below 10 °C (50 °F).[18][17]
Trophozoite stage
[edit]The trophozoite stage is the infective phase for humans, during which the organism can actively feed and replicate. The trophozoite attaches to the olfactory epithelium, follows the axons of olfactory receptor neurons through the cribriform plate in the nasal cavity, and enters the brain. This reproductive stage of the protozoan organism transforms around 25 °C (77 °F), and thrives best at approximately 42 °C (108 °F), multiplying through binary fission.
Trophozoites are characterized by a nucleus surrounded by a flexible membrane. They move via pseudopodia, extending parts of their cell membrane (pseudopods) and filling them with protoplasm to facilitate locomotion. Pseudopods form in the direction of movement. In their free-living state, trophozoites feed on bacteria. In tissues, they appear to phagocytize (enclose and digest) red blood cells and cause tissue damage either through the release of cytolytic substances or by direct cell-to-cell contact using cytolytic membrane proteins.[18]
As trophozoites, Naegleria fowleri may develop approximately 1 to 12 structures on their membrane known as amoebastomes (amorphous cytostomes), also referred to as "suckers" or "food cups," which they use for feeding in a manner similar to trogocytosis.[19]
Flagellate stage
[edit]The flagellate stage of Naegleria fowleri is pear-shaped and biflagellate (with two flagella). This stage can be inhaled into the nasal cavity, typically during activities such as swimming or diving. The flagellate form develops when trophozoites are exposed to a change in ionic strength in the fluid it is in, such as being placed in distilled water. The flagellate form does not exist in human tissue, but can be present in the cerebrospinal fluid. Once inside the nasal cavity, the flagellated form transforms into a trophozoite within a few hours.[18]
Ecology
[edit]Naegleria fowleri, an excavate, inhabits soil and water. It is sensitive to drying and acidic conditions, and cannot survive in seawater. The amoeba thrives at moderately elevated temperatures, making infections more likely during summer months. N. fowleri is a facultative thermophile, capable of growing at temperatures up to 46 °C (115 °F).[12] Warm freshwater with an ample supply of bacteria as food provides a suitable habitat for amoebae. Locations where many amoebic infections have occurred include artificial bodies of water, disturbed natural habitats, areas with soil, and unchlorinated or unfiltered water.
N. fowleri appears to flourish during periods of disturbance. The "flagellate-empty" hypothesis suggests that Naegleria's success may stem from decreased competition when thermosensitive protozoal fauna do not survive changes in temperature. In other words, N. fowleri thrives when other predators consuming its food supply are absent. This hypothesis implies that human disturbances, such as thermal pollution, increase the abundance of N. fowleri by eliminating its resource competitors. Amoeboflagellates have a motile flagellate stage that aids in dispersal, that is advantageous in environments cleared of competing organisms.
Pathogenicity
[edit]
N. fowleri may cause a usually fatal infection of the brain called naegleriasis, primary amoebic meningoencephalitis (PAM), amoebic encephalitis/meningitis, or simply Naegleria infection. Infections most often occur when water containing N. fowleri is inhaled through the nose (aspirated), where it then enters the nasal and olfactory nerve tissue, travelling to the brain through the cribriform plate.[20] Swallowing contaminated water does not cause infection by N. fowleri.[21] Infections typically occur after swimming in warm-climate freshwater, although there have been cases in cooler climates such as Minnesota, US.[22] In rare cases, infection has been caused by nasal or sinus rinsing with contaminated water in a nasal rinsing device such as a neti pot.[11] These account for 9% of worldwide cases.[23]
N. fowleri normally eat bacteria, but during human infections, the trophozoites consume astrocytes and neurons. The reason why N. fowleri passes across the cribriform plate is not known, but the neurotransmitter acetylcholine has been suggested as a stimulus precipitating the action, as a structural homolog of animal CHRM1 has been shown to be present in Naegleria and Acanthamoeba.[24]
The disease presents diagnostic challenges to medical professionals as early symptoms can be mild. 16% of cases presented with early flu-like symptoms only.[23] Symptoms may also appear similar to a viral or bacterial meningitis which may delay correct diagnosis and treatment.[25] Most cases have been diagnosed post-mortem following a biopsy of patient brain tissue.[26] It takes one to twelve days, median five, for symptoms to appear after nasal exposure to N. fowleri flagellates.[27] Symptoms may include headache, fever, nausea, vomiting, loss of appetite, altered mental state, coma, drooping eyelid, blurred vision, and loss of the sense of taste.[28] Later symptoms may include stiff neck, confusion, lack of attention, loss of balance, seizures, and hallucinations. Once symptoms begin to appear, the patient usually dies within two weeks. N. fowleri is not contagious; an infected person cannot transmit the infection.
Primary amoebic meningoencephalitis is classified as a rare disease in the United States as it affects fewer than 200,000 people.[29] From 2013 to 2022, 29 infections were reported in the US, which compares with about 4,000 annual deaths by drowning.[30] It is so rare that individual cases are often reported internationally, with 381 cases reported globally.[23][31] The true number of cases is likely to be higher than those reported due to problems relating to diagnosis, access to diagnostic testing and a lack of surveillance.[23]
Animals may be infected by Naegleria fowleri. This is rarely observed, although it may occur and be overlooked. Experimentally, mice, guinea pigs, and sheep have been infected, and there have been reports of South American tapirs and cattle contracting PAM.[32]
Treatment
[edit]The core antimicrobial treatment consists of the antifungal drug amphotericin B,[33] which inhibits the pathogen by binding to its cell membrane sterols, causing cell membrane disruption and pathogen death;[34] however, even with this treatment, the fatality rate is greater than 97%.[30][35] New treatments are being sought.[30][36] Miltefosine, an antiparasitic drug that inhibits the pathogen via disrupting its cell survival signal pathway PI3K/Akt/mTOR,[34] has been used in a few cases with mixed results.[37] Other treatments include dexamethasone and therapeutic hypothermia,[38] that may be utilised to reduce inflammation. Therapeutic hypothermia reduces the body's temperature to a hypothermic state[39] to prevent further brain injury resulting from hyper inflammation and increased intracranial pressure.[40]
A key factor to effective treatment is the speed of diagnosis. Naegleriasis is rare, and is often not considered as a likely diagnosis; therefore, the clinical laboratory's identification of the microorganism may be the first time an amoebic etiology is considered. Rapid identification can help to avoid delays in diagnosis and therapy. Amoeba cultures and real-time polymerase chain reaction (PCR) studies for N. fowleri are diagnostic of PAM, but they are not readily available at most institutions, and would have to be carried out at a reference laboratory. The time of presentation of the patient may affect the identification of the microorganism also, as PAM has an incubation period ranging from 1 to 12 days.[30] The clinical signs of PAM are similar to bacterial and viral meningitis, including fever, neck stiffness, and severe headaches. Symptoms can progress to prolonged nausea, vomiting, and even seizures. The disease can progress to acute hemorrhagic necrotizing meningoencephalitis. After symptoms start the patient typically dies within 1 to 18 days, typically about 5 days.[30] A variable delay in treatment can be secondary to time intervals in multiple stages of care, including exposure to exhibition of symptoms; arrival for treatment at a health care facility; workup of the diagnosis (initial diagnosis of likely bacterial meningitis); and finally, from diagnosis to initiation of recommended therapy. Successful treatment of PAM is rare; treatment can only be attempted after correct diagnosis, which relies on rapid recognition of the microorganism by medical technologists and pathologists. It is critical that medical technologists consistently provide timely CSF evaluation, explore the diagnosis of PAM, and look for amoebae in the setting of meningitis, especially in summer.[41]
Preventing human infection
[edit]A large proportion of reported cases of infection had a history of water exposure, 58% from swimming or diving, 16% from bathing, 10% from water sports such as jet skiing, water-skiing and wakeboarding and 9% from nasal irrigation.[23] Methods of infection prevention therefore focus on precautions to be taken around water to prevent water entering the nose, particularly during warmer weather. Wearing a nose clip when swimming may help to prevent contaminated water travelling up the nasal cavity. Keeping the head above water and not jumping or diving into warm fresh water may also prevent contaminated water from going up the nose. Swimmers should also avoid digging or stirring up sediment at the bottom of lakes, ponds and rivers as this is where amoebae are most likely to live.[42][43]
When irrigating sinuses or taking part in ritual cleansing of the nasal cavity, it is advised to use boiled or distilled water.[44]
Presence of Naegleria fowleri in the United States
[edit]Naegleria fowleri in Grand Teton National Park
[edit]
A study was conducted on five separate hot springs found in Grand Teton National Park in search for the presence of Naegleria fowleri over July 2016 to March 2017. The sites consisted of one spring from the Polecat Hot Springs, 3 springs from Huckleberry Hot Springs, and one spring from the Kelly Warm Springs from which a sample of water, sediment, and biofilm was taken in the months of July of 2016, November of 2016, and March of 2017. It was found that Naegleria fowleri was present in every hot spring in at least one of the three sample categories taken in the study. A notable result is that the samples taken from the Kelly Warm springs had a very low amount of detected Naegleria fowleri in the sediment and no detected presence in the water or biofilm samples. The Kelly Warm springs also had the lowest mean water temperature at 27.5°C. The springs with the highest mean temperatures were the first of the Huckleberry Hot Springs at 41.8°C and the Polecat Hot Spring at 42.3°C. The first Huckleberry Hot Spring and the Polecat Hot Spring were found to harbor Naegleria fowleri in their water for every month a sample was taken.[45][46]
Cases in Pakistan and India
[edit]This section needs additional citations for verification. (January 2024) |
Cases of infection in Pakistan account for 11% of reported cases globally.[23] In Pakistan, the number of reported cases has surpassed the global total due to insufficient healthcare infrastructure and limited awareness of Naegleria fowleri. As a result, only a small fraction of cases are correctly identified as primary amebic meningoencephalitis (PAM), with the majority of cases misdiagnosed as viral meningitis.
For the first time in Pakistan, N. fowleri genotype has been identified as type-2. Phylogenetic analysis showed that N. fowleri isolate from Pakistan is among the latest descendants, i.e., evolved later in life.[47]
In 2023, ten deaths were reported as a result of Naegleria. Four cases of this deadly infection have been reported in Pakistan as of 2024.[48]
India saw significant rise in primary amoebic meningoencephalitis (PAM) cases in 2024. In Kerala, four cases were reported over two months, leading to three deaths, while a 14-year-old boy from Kozhikode made a rare recovery, defying the nearly 97% mortality rate.[49] His recovery was attributed to early detection and treatment using miltefosine. Meanwhile, Kolkata recorded six cases in a year.[50] This increase has raised concerns, particularly given the infection’s 97% fatality rate. Health experts call for comprehensive studies to determine the reasons behind this sudden rise.[citation needed]
See also
[edit]- Acanthamoeba – an amoeba that can cause amoebic keratitis and encephalitis in humans
- Balamuthia mandrillaris – an amoeba that is the cause of (often fatal) granulomatous amoebic meningoencephalitis
- Entamoeba histolytica – an amoeba that is the cause of amoebiasis, or amoebic dysentery
- Leptospira – a zoonotic bacteria that causes leptospirosis
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Necrotizing fasciitis – the "flesh-eating disease", caused by certain types of bacteria
- Toxoplasma gondii – cat-carried protozoan that causes the disease toxoplasmosis
- Vibrio vulnificus – warm saltwater infectious bacteria
References
[edit]- ^ Schuster, Frederick L.; Visvesvara, Govinda S. (2004). "Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals". International Journal for Parasitology. 34 (9): 1001–1027. doi:10.1016/j.ijpara.2004.06.004. PMID 15313128.
- ^ "Texas residents warned of tap water tainted with brain-eating microbe". The Guardian. Associated Press. 26 September 2020. Archived from the original on 27 September 2020. Retrieved 27 September 2020.
- ^ Wellings, F. M.; Amuso, P. T.; Chang, S. L.; Lewis, A. L. (1977). "Isolation and identification of pathogenic Naegleria from Florida lakes". Appl Environ Microbiol. 34 (6): 661–7. Bibcode:1977ApEnM..34..661W. doi:10.1128/AEM.34.6.661-667.1977. PMC 242727. PMID 596870.
- ^ Sheehan, Kathy B.; Fagg, Jennifer A.; Ferris, Michael J.; Henson, Joan M. (2003). "PCR Detection and Analysis of the Free-Living Amoeba Naegleria in Hot Springs in Yellowstone and Grand Teton National Parks". Applied and Environmental Microbiology. 69 (10): 5914–5918. Bibcode:2003ApEnM..69.5914S. doi:10.1128/AEM.69.10.5914-5918.2003. PMC 201221. PMID 14532044.
- ^ Sykora, J. L.; Keleti, G.; Martinez, A. J. (1983). "Occurrence and pathogenicity of Naegleria fowleri in artificially heated waters". Appl Environ Microbiol. 45 (3): 974–9. Bibcode:1983ApEnM..45..974S. doi:10.1128/AEM.45.3.974-979.1983. PMC 242399. PMID 6847189.
- ^ Marciano-Cabral, Francine; MacLean, Rebecca; Mensah, Alex; LaPat-Polasko, Laurie (2003). "Identification of Naegleria fowleri in Domestic Water Sources by Nested PCR". Applied and Environmental Microbiology. 69 (10): 5864–5869. Bibcode:2003ApEnM..69.5864M. doi:10.1128/AEM.69.10.5864-5869.2003. PMC 201236. PMID 14532037.
- ^ Yoder, J. S.; Eddy, B. A.; Visvesvara, G. S.; Capewell, L.; Beach, M. J. (2009). "The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008". Epidemiology and Infection. 138 (7): 968–975. doi:10.1017/S0950268809991014. PMID 19845995. S2CID 7828942.
- ^ Maclean, RebeccaC.; Richardson, DennisJ.; LePardo, Robin; Marciano-Cabral, Francine (2004). "The identification of Naegleria fowleri from water and soil samples by nested PCR". Parasitology Research. 93 (3): 211–217. doi:10.1007/s00436-004-1104-x. PMID 15138806. S2CID 5972631.
- ^ NCCEH (March 1, 2011). "Pool chlorination and closure guidelines".
- ^ Yoder, J. S.; Straif-Bourgeois, S.; Roy, S. L.; Moore, T. A.; Visvesvara, G. S.; Ratard, R. C.; Hill, V. R.; Wilson, J. D.; Linscott, A. J.; Crager, R.; Kozak, N. A.; Sriram, R.; Narayanan, J.; Mull, B.; Kahler, A. M.; Schneeberger, C.; da Silva, A. J.; Poudel, M.; Baumgarten, K. L.; Xiao, L.; Beach, M. J. (2012). "Primary Amebic Meningoencephalitis Deaths Associated With Sinus Irrigation Using Contaminated Tap Water". Clinical Infectious Diseases. 55 (9): e79–e85. doi:10.1093/cid/cis626. PMC 11307261. PMID 22919000.
- ^ a b "Naegleria fowleri — Primary Amebic Meningoencephalitis (PAM): Ritual Nasal Rinsing & Ablution". www.cdc.gov. CDC. 2023-05-03. Archived from the original on 2022-11-30.
- ^ a b "Naegleria fowleri — Primary Amebic Meningoencephalitis (PAM): General Information". Centers for Disease Control and Prevention (CDC). 2023-05-03. Archived from the original on 2018-07-28.
- ^ Fowler, M.; Carter, R. F. (September 1965). "Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report". The BMJ. 2 (5464): 740–2. doi:10.1136/bmj.2.5464.734-a. PMC 1846173. PMID 5825411.
- ^ "The discovery of amoebic meningitis in Northern Spencer Gulf towns". samhs.org. South Australian Medical Heritage Society Inc. Archived from the original on October 22, 2022. Retrieved August 15, 2019.
- ^ Laseke I, Korte J, Lamendella R, Kaneshiro ES, Marciano-Cabral F, Oerther DB (January 2010). "Identification of Naegleria fowleri in warm ground water aquifers". Journal of Environmental Quality. 39 (1): 147–153. Bibcode:2010JEnvQ..39..147L. doi:10.2134/jeq2009.0062. PMC 6844256. PMID 20048302.
- ^ "Brain-eating-amoeba". WebMD. Archived from the original on 21 August 2016. Retrieved 1 July 2015.
- ^ a b Chang, S.L. (1978). "Resistance of pathogenic Naegleria to some common physical and chemical agents". Applied and Environmental Microbiology. 35 (2): 368–375. Bibcode:1978ApEnM..35..368C. doi:10.1128/AEM.35.2.368-375.1978. PMC 242840. PMID 637538.
- ^ a b c d Marciano-Cabral, F (1988). "Biology of Naegleria spp". Microbiological Reviews. 52 (1): 114–133. doi:10.1128/MMBR.52.1.114-133.1988. PMC 372708. PMID 3280964.
- ^ John, D T; Cole, T B; Marciano-Cabral, F M (January 1984). "Sucker-like structures on the pathogenic amoeba Naegleria fowleri". Applied and Environmental Microbiology. 47 (1): 12–14. Bibcode:1984ApEnM..47...12J. doi:10.1128/aem.47.1.12-14.1984. ISSN 0099-2240. PMC 239603. PMID 6696410.
- ^ Baig, AM (Aug 2015). "Pathogenesis of amoebic encephalitis: Are the amoebae being credited to an 'inside job' done by the host immune response?". Acta Trop. 148: 72–76. doi:10.1016/j.actatropica.2015.04.022. PMID 25930186.
- ^ "Primary Amebic Meningoencephalitis (PAM) - Naegleria fowleri | Parasites | CDC". www.cdc.gov. 2019-06-24. Archived from the original on 2019-01-29. Retrieved 2020-10-16.
- ^ "Naegleria and Amebic Meningoencephalitis – Minnesota Dept. of Health". www.health.state.mn.us. Archived from the original on 2022-12-28. Retrieved 2020-10-16.
- ^ a b c d e f Gharpure, Radhika; Bliton, John; Goodman, Alexandra; Ali, Ibne Karim M; Yoder, Jonathan; Cope, Jennifer R (2021-07-01). "Epidemiology and Clinical Characteristics of Primary Amebic Meningoencephalitis Caused by Naegleria fowleri : A Global Review". Clinical Infectious Diseases. 73 (1): e19–e27. doi:10.1093/cid/ciaa520. ISSN 1058-4838. PMC 8739754. PMID 32369575.
- ^ Baig, AM (Aug 2016). "Primary Amoebic Meningoencephalitis: Neurochemotaxis and Neurotropic Preferences of Naegleria fowleri". ACS Chem Neurosci. 7 (8): 1026–1029. doi:10.1021/acschemneuro.6b00197. PMID 27447543.
- ^ "Illness and Symptoms | Naegleria fowleri | CDC". www.cdc.gov. 2023-05-03. Retrieved 2024-03-12.
- ^ Güémez, Andrea; García, Elisa (2021-09-06). "Primary Amoebic Meningoencephalitis by Naegleria fowleri: Pathogenesis and Treatments". Biomolecules. 11 (9): 1320. doi:10.3390/biom11091320. ISSN 2218-273X. PMC 8469197. PMID 34572533.
- ^ "Naegleria fowleri – Primary Amebic Meningoencephalitis (PAM): Illness & Symptoms". Centers for Disease Control and Prevention (CDC). May 3, 2023. Archived from the original on May 11, 2020.
- ^ "Brain-Eating Amoeba (Naegleria Fowleri): FAQ, Symptoms, Treatment". WebMD. 2021-09-29. Archived from the original on 2022-12-28.
- ^ "General Information | Naegleria fowleri | CDC". www.cdc.gov. 2023-05-03. Retrieved 2024-03-12.
- ^ a b c d e "Frequently asked questions about Naegleria fowleri, commonly known as the "brain-eating ameba"". CDC.gov. Centers for Disease Control and Prevention (CDC). May 3, 2023. Archived from the original on May 13, 2020.
- ^ Helmore, Edward (19 September 2023). "Arkansas child dies of rare brain-eating amoeba after playing at country club". The Guardian (UK).
- ^ "Naegleria Fowleri in Animals" (PDF). ldh.la.gov. Infectious Disease Epidemiology Section, Office of Public Health, Louisiana Dept of Health & Hospitals. 25 September 2013. Archived (PDF) from the original on 5 February 2023.
- ^ Subhash Chandra Parija (Nov 23, 2015). "Naegleria Infection Treatment & Management". Medscape. Archived from the original on November 13, 2019.
- ^ a b Asbill, Scott; Virga, Kris (2015). "Naegleria Fowleri: Pathogenesis, Diagnosis, and Treatment Options". Antimicrobial Agents and Chemotherapy. 59 (11): 6677–6681. doi:10.1128/AAC.01293-15. PMC 4604384. PMID 26259797.
- ^ Cetin, N; Blackall, D (April 2012). "Naegleria fowleri meningoencephalitis". Blood. 119 (16): 3658. doi:10.1182/blood-2011-06-353136. PMID 22645743. S2CID 8912435.
- ^ Wessel, Lindzi (22 July 2016). "Scientists scour the globe for a drug to kill deadly brain-eating amoeba". STAT. Archived from the original on 6 October 2020.
- ^ Wessel, Linda (16 September 2016). "A life-saving drug that treats a rare infection is almost impossible to find". Business Insider. Archived from the original on 19 September 2016.
- ^ "Treatment | Naegleria fowleri | CDC". www.cdc.gov. 2023-05-03. Retrieved 2024-03-12.
- ^ "Treatment | Naegleria fowleri | CDC". www.cdc.gov. 2023-05-03. Retrieved 2024-03-12.
- ^ Güémez, Andrea; García, Elisa (2021-09-06). "Primary Amoebic Meningoencephalitis by Naegleria fowleri: Pathogenesis and Treatments". Biomolecules. 11 (9): 1320. doi:10.3390/biom11091320. ISSN 2218-273X. PMC 8469197. PMID 34572533.
- ^ Pugh, J. Jeffrey; Levy, Rebecca A. (2016-09-21). "Naegleria fowleri: Diagnosis, Pathophysiology of Brain Inflammation, and Antimicrobial Treatments". ACS Chemical Neuroscience. 7 (9): 1178–1179. doi:10.1021/acschemneuro.6b00232. PMID 27525348.
- ^ CDC (2022-08-19). "Naegleria fowleri infections are rare". Centers for Disease Control and Prevention. Retrieved 2024-03-12.
- ^ "Pathogen and Environment | Naegleria fowleri | CDC". www.cdc.gov. 2022-10-18. Retrieved 2024-03-12.
- ^ "Ritual Nasal Rinsing & Ablution | Naegleria fowleri | CDC". www.cdc.gov. 2023-05-03. Retrieved 2024-03-12.
- ^ Barnhart, Elliott; Kinsey, Stacy; Wright, Peter; Caldwell, Sara; Hill, Vince; Kahler, Amy; Mattioli, Mia; Cornman, Robert; Iwanowicz, Deborah; Eddy, Zachary; Halonen, Sandra; Mueller, Rebecca; Peyton, Brent; Geoffrey, Puzon (January 9, 2024). "Naegleria fowleri Detected in Grand Teton National Park Hot Springs". ACS EST Water 2024. 4 (1): 628–637.
- ^ Sheehan, Kathy; Fagg, Jennifer; Ferris, Michael; Henson, Joan (1 October 2023). "PCR Detection and Analysis of the Free-Living Amoeba Naegleria in Hot Springs in Yellowstone and Grand Teton National Parks". Applied and Environmental Microbiology. 69 (10) – via OU Libraries.
- ^ Aurongzeb, Muhammad; Rashid, Yasmeen; Ahmed Naqvi, Syed Habib; Khatoon, Ambrina; Abdul Haq, Sadia; Azim, Mohammad Kamran; Kaleem, Imdad; Bashir, Shahid (2022). "Naegleria fowleri from Pakistan Has Type-2 Genotype". Iranian Journal of Parasitology. 17 (1): 43–52. doi:10.18502/ijpa.v17i1.9015. ISSN 1735-7020. PMC 9375727. PMID 36046566.
- ^ Reporter, The Newspaper's Staff (2024-09-03). "Brain-eating amoeba claims life of teenager". DAWN.COM. Retrieved 2024-09-03.
- ^ Jayanth, A. S. (2024-06-30). "Unhygienic water, mercury rise may be behind 'brain-eating amoeba' cases in Kerala". The Hindu. ISSN 0971-751X. Retrieved 2024-08-16.
- ^ "6 brain-eating amoeba cases in a year". The Times of India. 2024-07-23. ISSN 0971-8257. Retrieved 2024-08-16.
